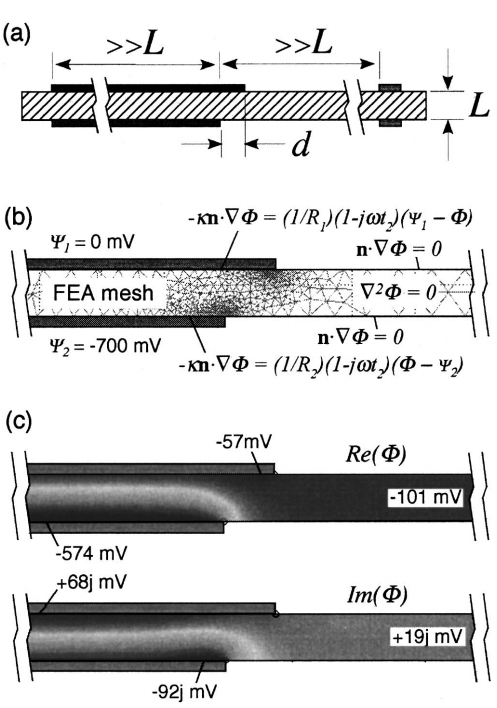
Abstract
To separate anode and cathode contributions to the impedance of a thin solid electrolyte cell, many workers place a reference
electrode on the surface of the inactive electrolyte, coplanar with the working electrode. This practice is evaluated theoretically
using finite-element calculations of the real and imaginary potential distributions in the electrolyte under the conditions of ac
impedance. The results of this analysis show that minor errors in the alignment of the anode and cathode can create significant
errors in the measured half-cell overpotential. These errors involve not only quantitative scaling factors, but also crosscontamination
of anode and cathode frequency response in the measured half-cell impedances. Even if electrodes are perfectly
aligned, differences between the anode and cathode kinetics and/or frequency response may cause inherent distortion of the
impedance, including frequency dispersion and inductive artifacts. We evaluate two approaches (pellets and microelectrode arrays)
that can be used to avoid ambiguities.
# Graphical Abstract