Abstract
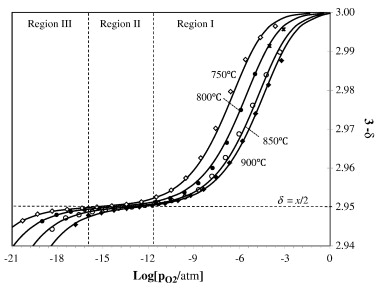
The equilibrium oxygen nonstoichiometry, δ, for the mixed ionic and electronic conducting perovskite La0.9Ca0.1FeO3 − δ (LCF91) was measured for oxygen partial
pressures, pO2, between 10− 3 and 10− 21 atm and temperatures of 750 to 900 °C using a tubular, two-probe Pt|YSZ|LCF coulometric titration cell. XRD experiments
confirmed that LCF91 remains a single phase perovskite under the reducing conditions of a fuel electrode, and reveal no evidence of reaction with the zirconia
electrolyte. The pO2-nonstoichiometry relationship can be interpreted using a dilute-solution point-defect model involving oxygen vacancies, electrons, and
electron holes.
Graphical Abstract